加拿大化學競賽(Canadian Chemistry Contest)競賽竟然考到四種分子間作用力?學alevel是不會(hui) 告訴你的。一般alevel隻學到三種分子間作用力:
而在CCC考綱明確要求我們(men) 需要掌握4種,並且能夠利用這些分子間作用力判斷熔沸點和溶解度。
5.11 explain the effect of solutes on the melting point of solid water, using intermolecular forces
根據我在《加拿大化學競賽CCC複習(xi) 指導》給大家總結的內(nei) 容,跟大家補充一下這方麵的知識:
Intermolecular forces的定義(yi) :
Intermolecular forces are attractive forces between molecules. They are largely responsible for the observed boiling points and solubility properties of molecules.

1. 倫(lun) 敦力,也叫色散力:
1). London Dispersion: Attraction between molecules that form due to a temporary dipole on one molecule inducing a temporary dipole in neighboring molecules.
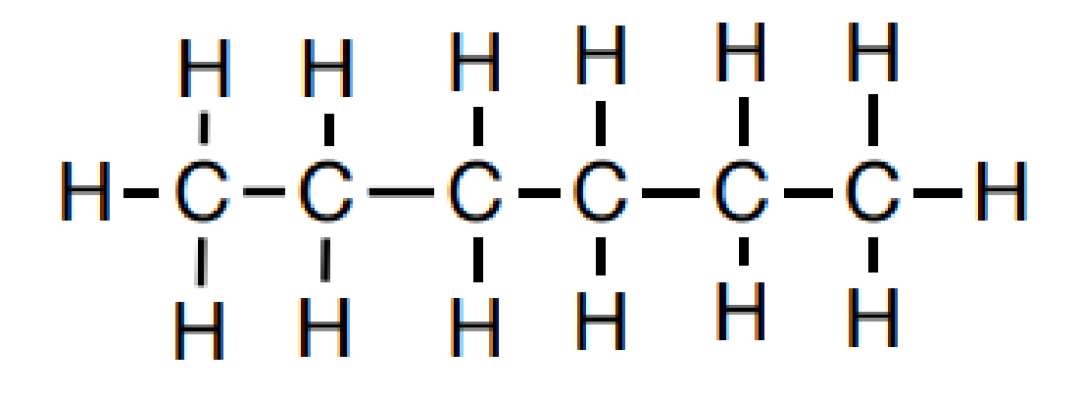
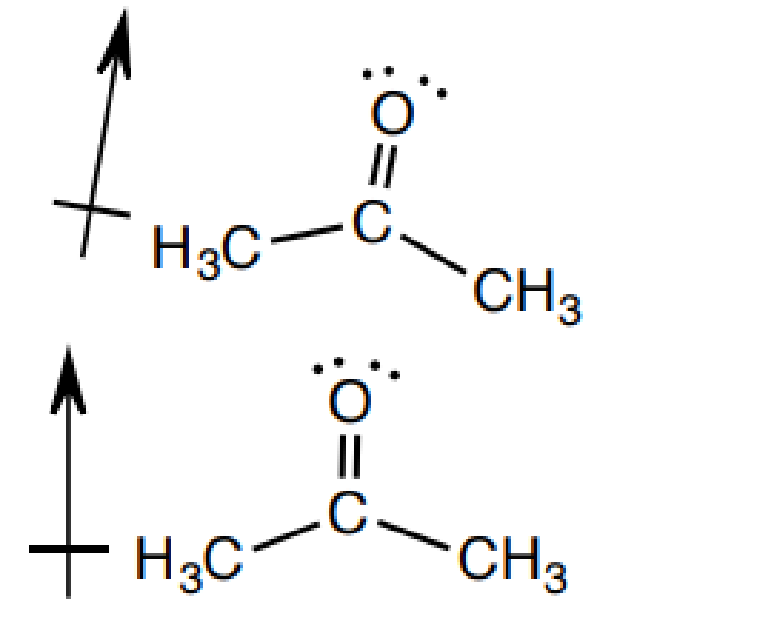
2.偶極-偶極 作用
2). Dipole-Dipole: Attraction between two polar molecules (dipoles)
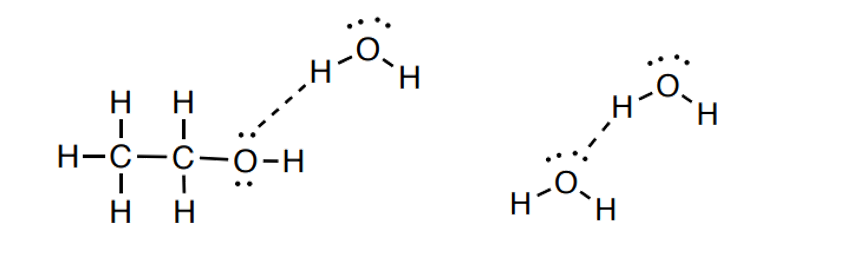
3. 氫鍵
3). Hydrogen Bonding: Attraction between two polar molecules, specifically one molecule having a H bonded directly to an electronegative atom (eg. H-O-, H-N-, HF), and the other having a non-bonding electron pair on an electronegative atom.
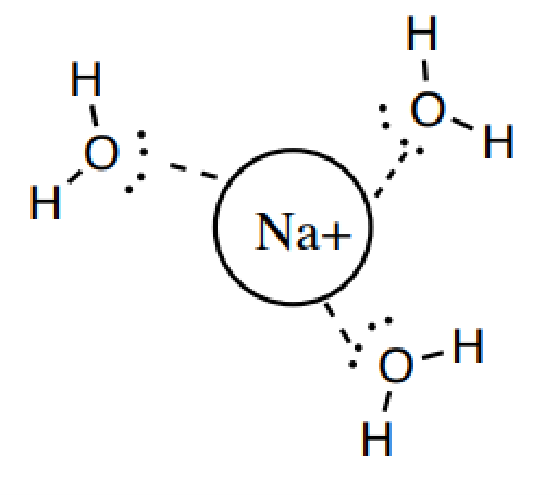
4. 離子偶極作用(這是我們(men) 今天提到的!)
4).Ion-Dipole: Attraction between an ion and a polar molecule.
這幾種力相對強弱如下:
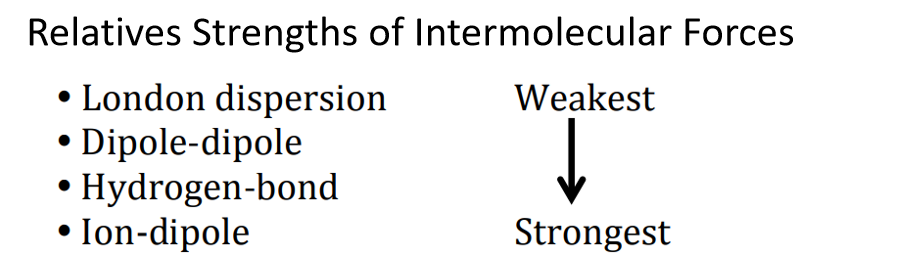
如果你需要判斷他們(men) 熔沸點的大小,除了知道這些作用力還需要了解這些“趨勢(trends)”:
-
Between two molecules of similar mass, the one with the stronger type of intermolecular force has a higher boiling point.

2. Between two nonpolar molecules of similar mass, the more extended molecule will have the higher boiling point (more extended amore surface area for London dispersion interaction).
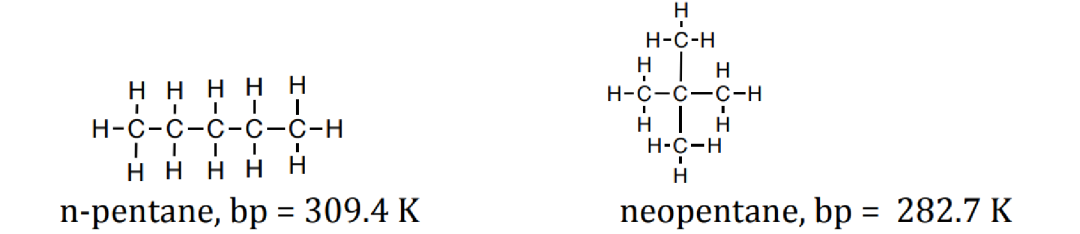
3. Between two nonpolar molecules of different masses, the larger molecule will have the higher boiling point (larger molecule → more electrons → more polarizability → more London dispersion forces)

相關(guan) 真題我總結在《加拿大化學思維挑戰CCC分類真題》專(zhuan) 輯裏麵:
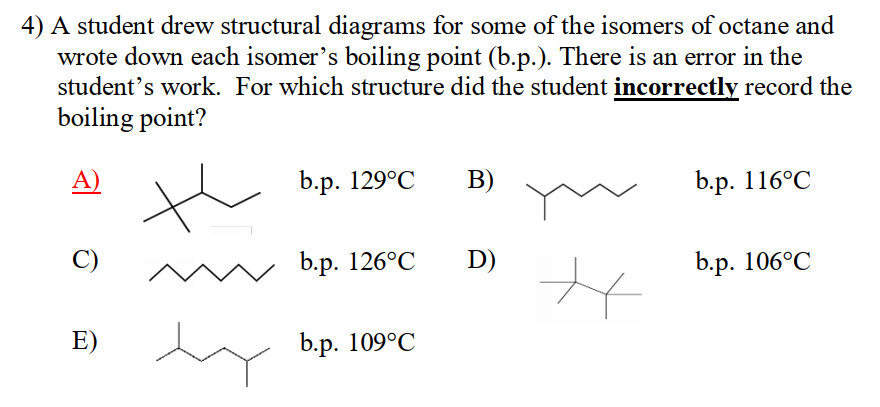
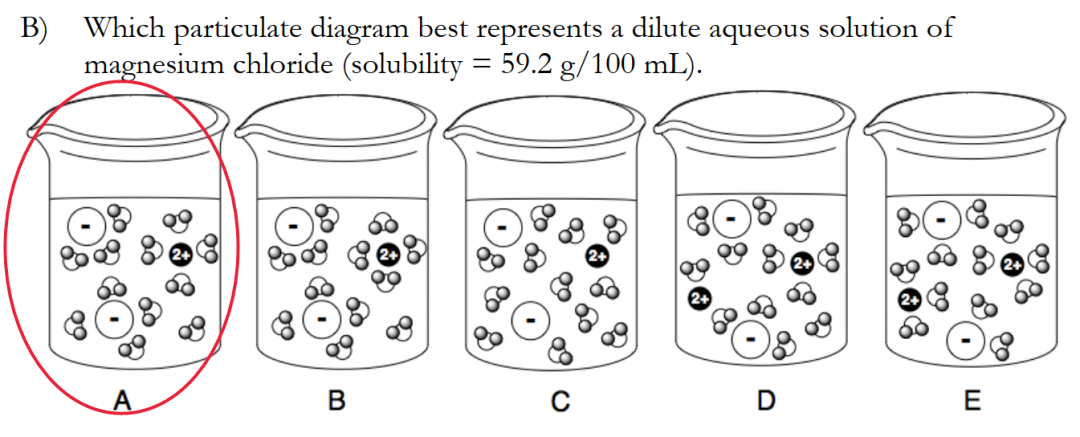













評論已經被關(guan) 閉。